Parkinson's disease also known as Parkinson disease, is a degenerative disorder of the central nervous system that often impairs the sufferer's motor skills and speech. Parkinson's disease belongs to a group of conditions called movement disorders. It is characterized by muscle rigidity, tremor, a slowing of physical movement (bradykinesia), and in extreme cases, a loss of physical movement (akinesia). The primary symptoms are the results of decreased stimulation of the motor cortex by the basal ganglia, normally caused by the insufficient formation and action of dopamine, which is produced in the dopaminergic neurons of the brain. Secondary symptoms may include high level cognitive dysfunction and subtle language problems. PD is both chronic and progressive.
Pages
▼
Myasthenia gravis
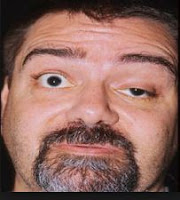
Myasthenia gravis is a neuromuscular disease leading to fluctuating muscle weakness and fatigability during simple activities. Weakness is typically caused by circulating antibodies that block acetylcholine receptors at the post-synaptic neuromuscular junction, inhibiting the stimulative effect of the neurotransmitter acetylcholine. Myasthenia is treated with immunosuppressants, cholinesterase inhibitors and, in selected cases, thymectomy.
Cholinergic hypothesis Of Alzheimer's disease
The oldest, on which most currently available drug therapies are based, is the cholinergic hypothesis, which proposes that AD is caused by reduced synthesis of the neurotransmitter acetylcholine. The cholinergic hypothesis has not maintained widespread support, largely because medications intended to treat acetylcholine deficiency have not been very effective. Other cholinergic effects have also been proposed, for example, initiation of large-scale aggregation of amyloid, leading to generalised neuroinflammation.
Advanced Of Alzheimer's disease
 During the final stage of AD, the person is completely dependent upon caregivers. Language is reduced to simple phrases or even single words, eventually leading to complete loss of speech. Despite the loss of verbal language abilities, people can often understand and return emotional signals. Although aggressiveness can still be present, extreme apathy and exhaustion are much more common results. People with AD will ultimately not be able to perform even the simplest tasks without assistance. Muscle mass and mobility deteriorate to the point where they are bedridden, and they lose the ability to feed themselves. AD is a terminal illness, with the cause of death typically being an external factor, such as infection of pressure ulcers or pneumonia, not the disease itself.
During the final stage of AD, the person is completely dependent upon caregivers. Language is reduced to simple phrases or even single words, eventually leading to complete loss of speech. Despite the loss of verbal language abilities, people can often understand and return emotional signals. Although aggressiveness can still be present, extreme apathy and exhaustion are much more common results. People with AD will ultimately not be able to perform even the simplest tasks without assistance. Muscle mass and mobility deteriorate to the point where they are bedridden, and they lose the ability to feed themselves. AD is a terminal illness, with the cause of death typically being an external factor, such as infection of pressure ulcers or pneumonia, not the disease itself.Moderate Of Alzheimer's disease
Progressive deterioration eventually hinders independence, with subjects being unable to perform most common activities of daily living. Speech difficulties become evident due to an inability to recall vocabulary, which leads to frequent incorrect word substitutions (paraphasias). Reading and writing skills are also progressively lost. Complex motor sequences become less coordinated as time passes and AD progresses, so the risk of falling increases. During this phase, memory problems worsen, and the person may fail to recognise close relatives. Long-term memory, which was previously intact, becomes impaired.
Behavioural and neuropsychiatric changes become more prevalent. Common manifestations are wandering, irritability and labile affect, leading to crying, outbursts of unpremeditated aggression, or resistance to caregiving. Sundowning can also appear. Approximately 30% of people with AD develop illusionary misidentifications and other delusional symptoms. Subjects also lose insight of their disease process and limitations (anosognosia). Urinary incontinence can develop. These symptoms create stress for relatives and caretakers, which can be reduced by moving the person from home care to other long-term care facilities.
Pre-dementia Of Alzheimer's disease
The first symptoms are often mistakenly attributed to ageing or stress Detailed neuropsychological testing can reveal mild cognitive difficulties up to eight years before a person fulfils the clinical criteria for diagnosis of AD..These early symptoms can affect the most complex daily living activities. The most noticeable deficit is memory loss, which shows up as difficulty in remembering recently learned facts and inability to acquire new information.
Subtle problems with the executive functions of attentiveness, planning, flexibility, and abstract thinking, or impairments in semantic memory (memory of meanings, and concept relationships) can also be symptomatic of the early stages of AD. Apathy can be observed at this stage, and remains the most persistent neuropsychiatric symptom throughout the course of the disease. Depressive symptoms, irritability and reduced awareness of subtle memory difficulties also occur commonly. The preclinical stage of the disease has also been termed mild cognitive impairment, but whether this term corresponds to a different diagnostic stage or identifies the first step of AD is a matter of dispute.
Characteristics Of Alzheimer's disease
There Are Four Characteristics Of Alzheimer's disease
- Pre-dementia
- Early
- Moderate
- Advanced
Early Of Alzheimer's disease
 In people with AD the increasing impairment of learning and memory eventually leads to a definitive diagnosis. In a small portion of them, difficulties with language, executive functions, perception (agnosia), or execution of movements (apraxia) are more prominent than memory problems. AD does not affect all memory capacities equally. Older memories of the person's life (episodic memory), facts learned (semantic memory), and implicit memory (the memory of the body on how to do things, such as using a fork to eat) are affected to a lesser degree than new facts or memories.
In people with AD the increasing impairment of learning and memory eventually leads to a definitive diagnosis. In a small portion of them, difficulties with language, executive functions, perception (agnosia), or execution of movements (apraxia) are more prominent than memory problems. AD does not affect all memory capacities equally. Older memories of the person's life (episodic memory), facts learned (semantic memory), and implicit memory (the memory of the body on how to do things, such as using a fork to eat) are affected to a lesser degree than new facts or memories.
Language problems are mainly characterised by a shrinking vocabulary and decreased word fluency, which lead to a general impoverishment of oral and written language. In this stage, the person with Alzheimer's is usually capable of communicating basic ideas adequately. While performing fine motor tasks such as writing, drawing or dressing, certain movement coordination and planning difficulties (apraxia) may be present but they are commonly unnoticed. As the disease progresses, people with AD can often continue to perform many tasks independently, but may need assistance or supervision with the most cognitively demanding activities.
History Of Charcot–Marie–Tooth disease
 The disease is named after those who classically described it: Jean-Martin Charcot (1825–1893), his pupil Pierre Marie (1853–1940) ("Sur une forme particulière d'atrophie musculaire progressive, souvent familiale débutant par les pieds et les jambes et atteignant plus tard les mains", Revue médicale, Paris, 1886; 6: 97-138.), and Howard Henry Tooth (1856–1925) ("The peroneal type of progressive muscular atrophy", dissertation, London, 1886.)
The disease is named after those who classically described it: Jean-Martin Charcot (1825–1893), his pupil Pierre Marie (1853–1940) ("Sur une forme particulière d'atrophie musculaire progressive, souvent familiale débutant par les pieds et les jambes et atteignant plus tard les mains", Revue médicale, Paris, 1886; 6: 97-138.), and Howard Henry Tooth (1856–1925) ("The peroneal type of progressive muscular atrophy", dissertation, London, 1886.)Management Of Charcot–Marie–Tooth disease
Although there is no current standard treatment, the use of ascorbic acid has been proposed, and has shown some benefit in animal models. A clinical trial to determine the effectiveness of high doses of ascorbic acid (vitamin C) in treating humans with CMT type 1A has been conducted. The results of the trial upon children have shown that a high dosage intake of ascorbic acid is safe but the efficacy endpoints expected were not met. In 2010, a study published in the Journal Science indicated that scientists had identified those proteins that control the thickness of myelin sheath. This discovery is expected to open the avenue to new treatments in the coming years.
 The most important activity for patients with CMT is to maintain what movement, muscle strength, and flexibility they have. Therefore, physical therapy and moderate activity are recommended but overexertion should be avoided. A physiotherapist should be involved in designing an exercise program that fits a patient’s personal strengths and flexibility. Bracing can also be used to correct problems caused by CMT. Gait abnormalities can be corrected by the use of either articulated (hinged) or unarticulated, braces called AFOs (ankle-foot orthoses). These braces help control foot drop and ankle instability and often provide a better sense of balance for patients. Appropriate footwear is also very important for people with CMT, but they often have difficulty finding well-fitting shoes because of their high arched feet and hammer toes. Due to the lack of good sensory reception in the feet, CMT patients may also need to see a podiatrist for help in trimming nails or removing calluses that develop on the pads of the feet. A final decision a patient can make is to have surgery. Using a podiatrist or an orthopedic surgeon, patients can choose to stabilize their feet or correct progressive problems. These procedures include straightening and pinning the toes, lowering the arch, and sometimes, fusing the ankle joint to provide stability. CMT patients must take extra care to avoid falling because fractures take longer to heal in someone with an underlying disease process. Additionally, the resulting inactivity may cause the CMT to worsen.
The most important activity for patients with CMT is to maintain what movement, muscle strength, and flexibility they have. Therefore, physical therapy and moderate activity are recommended but overexertion should be avoided. A physiotherapist should be involved in designing an exercise program that fits a patient’s personal strengths and flexibility. Bracing can also be used to correct problems caused by CMT. Gait abnormalities can be corrected by the use of either articulated (hinged) or unarticulated, braces called AFOs (ankle-foot orthoses). These braces help control foot drop and ankle instability and often provide a better sense of balance for patients. Appropriate footwear is also very important for people with CMT, but they often have difficulty finding well-fitting shoes because of their high arched feet and hammer toes. Due to the lack of good sensory reception in the feet, CMT patients may also need to see a podiatrist for help in trimming nails or removing calluses that develop on the pads of the feet. A final decision a patient can make is to have surgery. Using a podiatrist or an orthopedic surgeon, patients can choose to stabilize their feet or correct progressive problems. These procedures include straightening and pinning the toes, lowering the arch, and sometimes, fusing the ankle joint to provide stability. CMT patients must take extra care to avoid falling because fractures take longer to heal in someone with an underlying disease process. Additionally, the resulting inactivity may cause the CMT to worsen.
The Charcot-Marie-Tooth Association classifies the chemotherapy drug vincristine as a "definite high risk" and states that "vincristine has been proven hazardous and should be avoided by all CMT patients, including those with no symptoms."
Diagnosis Of Charcot–Marie–Tooth disease
 CMT can be diagnosed through symptoms, through measurement of the speed of nerve impulses (electromyography), through biopsy of the nerve, and through DNA testing. DNA testing can give a definitive diagnosis, but not all the genetic markers for CMT are known. CMT is first noticed when someone develops lower leg weakness and foot deformities such as foot drop, hammertoes and high arches. But signs alone do not lead to diagnosis. Patients must be referred to a physician specialising in neurology or rehabilitation medicine. To see signs of muscle weakness the neurologist will ask patients to walk on their heels or to move part of their leg against an opposing force. In order to identify sensory loss the neurologist will test for deep tendon reflexes, such as the knee jerk, which are reduced or absent in CMT. The doctor will also ask about family history because CMT is hereditary. The lack of family history does not rule out CMT, but it will allow the doctor to rule out other causes of neuropathy such as diabetes or exposure to certain chemicals or drugs.
CMT can be diagnosed through symptoms, through measurement of the speed of nerve impulses (electromyography), through biopsy of the nerve, and through DNA testing. DNA testing can give a definitive diagnosis, but not all the genetic markers for CMT are known. CMT is first noticed when someone develops lower leg weakness and foot deformities such as foot drop, hammertoes and high arches. But signs alone do not lead to diagnosis. Patients must be referred to a physician specialising in neurology or rehabilitation medicine. To see signs of muscle weakness the neurologist will ask patients to walk on their heels or to move part of their leg against an opposing force. In order to identify sensory loss the neurologist will test for deep tendon reflexes, such as the knee jerk, which are reduced or absent in CMT. The doctor will also ask about family history because CMT is hereditary. The lack of family history does not rule out CMT, but it will allow the doctor to rule out other causes of neuropathy such as diabetes or exposure to certain chemicals or drugs.
In 2010, CMT was one of the first diseases where the genetic cause of a particular patient's disease was precisely determined by sequencing the whole genome of an affected individual. This was done by the scientists employed by the Charcot Marie Tooth Association (CMTA) Two mutations were identified in a gene, SH3TC2, known to cause CMT. Researchers then compared the affected patient's genome to the genomes of the patient's mother, father, and seven siblings with and without the disease. The mother and father each had one normal and one mutant copy of this gene, and had mild or no symptoms. The offspring that inherited two mutant genes presented fully with the disease. Sequencing the initial patient's whole genome cost $50,000, but researchers estimated that it would soon cost $5,000 and become common.
Charcot–Marie–Tooth disease is caused by mutations that cause defects in neuronal proteins. Nerve signals are conducted by an axon with a myelin sheath wrapped around it. Most mutations in CMT affect the myelin sheath, but some affect the axon.
The most common cause of CMT (70-80% of the cases) is the duplication of a large region on the short arm of chromosome 17 that includes the gene PMP22. Some mutations affect the gene MFN2, which codes for a mitochondrial protein. Cells contain separate sets of genes in their nucleus and in their mitochondria. In nerve cells, the mitochondria travel down the long axons. In some forms of CMT, mutated MFN2 causes the mitochondria to form large clusters, or clots, which are unable to travel down the axon towards the synapses. This prevents the synapses from functioning.
CMT is divided into the primary demyelinating neuropathies (CMT1, CMT3, and CMT4) and the primary axonal neuropathies (CMT2), with frequent overlap. Another cell involved in CMT is the Schwann cell, which creates the myelin sheath, by wrapping its plasma membrane around the axon in a structure that is sometimes compared to a Swiss roll.
Neurons, Schwann cells, and fibroblasts work together to create a working nerve. Schwann cells and neurons exchange molecular signals that regulate survival and differentiation. These signals are disrupted in CMT.
Demyelinating Schwann cells causes abnormal axon structure and function. They may cause axon degeneration, or they may simply cause axons to malfunction.
The myelin sheath allows nerve cells to conduct signals faster. When the myelin sheath is damaged, nerve signals are slower, and this can be measured by a common neurological test, electromyography. When the axon is damaged, on the other hand, this results in a reduced compound muscle action potential (CMAP).
Signs and symptoms Of Charcot–Marie–Tooth disease
Symptoms of CMT usually begin in late childhood or early adulthood. Some people do not experience symptoms until their early thirties or forties. Usually, the initial symptom is foot drop early in the course of the disease. This can also cause claw toe, where the toes are always curled. Wasting of muscle tissue of the lower parts of the legs may give rise to a "stork leg" or "inverted bottle" appearance. Weakness in the hands and forearms occurs in many people later in life as the disease progresses.
Loss of touch sensation in the feet, ankles and legs, as well as in the hands, wrists and arms is characteristic in various types of the disease. Early and late onset forms occur with 'on and off' painful spasmodic muscular contractions that can be disabling when the disease activates. High arched feet (pes cavus) are classically associated with the disorder. Sensory and proprioceptive nerves in the hands and feet are often damaged, while pain nerves are left intact. Overuse of an affected hand or limb can activate symptoms including numbness, spasm, and painful cramping.
Symptoms and progression of the disease can vary. Breathing can be affected in some; so can hearing, vision, as well as the neck and shoulder muscles. Scoliosis is common. Hip sockets can be malformed. Gastrointestinal problems can be part of CMT, as can chewing, swallowing, and speaking (due to atrophy of vocal cords). A tremor can develop as muscles waste. Pregnancy has been known to exacerbate CMT, as well as extreme emotional stress. Patients with CMT must avoid periods of prolonged immobility such as when recovering from a secondary injury as prolonged periods of limited mobility can drastically accelerate symptoms of CMT.
Neuropathic pain is often a symptom of CMT, though, like other symptoms of CMT, its presence and severity varies from case to case. For some people, pain can be significant to severe and interfere with daily life activities. However, pain is not experienced by all people with CMT. When pain is present as a symptom of CMT, it is comparable to that seen in other peripheral neuropathies, as well as Postherpetic neuralgia and Complex regional pain syndrome, among other diseases.
Nerve regeneration Of Neuron
 It has been demonstrated that neurogenesis can sometimes occur in the adult vertebrate brain, a finding that led to controversy in 1999. However, more recent studies of the age of human neurons suggest that this process occurs only for a minority of cells, and the overwhelming majority of neurons comprising the neocortex were formed before birth and persist without replacement.
It has been demonstrated that neurogenesis can sometimes occur in the adult vertebrate brain, a finding that led to controversy in 1999. However, more recent studies of the age of human neurons suggest that this process occurs only for a minority of cells, and the overwhelming majority of neurons comprising the neocortex were formed before birth and persist without replacement.
It is often possible for peripheral axons to regrow if they are severed. A report in Nature suggested that researchers had found a way to transform human skin cells into working nerve cells using a process called transdifferentiation in which "cells are forced to adopt new identities."
Axonal degeneration Of Neuron
Although most injury responses include a calcium influx signaling to promote resealing of severed parts, axonal injuries initially lead to acute axonal degeneration (AAD), which is rapid separation of the proximal and distal ends within 30 minutes of injury. Degeneration follows with swelling of the axolemma, and eventually leads to bead like formation. Granular disintegration of the axonal cytoskeleton and inner organelles occurs after axolemma degradation. Early changes include accumulation of mitochondria in the paranodal regions at the site of injury. Endoplasmic reticulum degrades and mitochondria swell up and eventually disintegrate. The disintegration is dependent on Ubiquitin and Calpain proteases (caused by influx of calcium ion), suggesting that axonal degeneration is an active process. Thus the axon undergoes complete fragmentation. The process takes about roughly 24 hrs in the PNS, and longer in the CNS. The signaling pathways leading to axolemma degeneration are currently unknown.
Demyelination Of Neuron
Demyelination is the act of demyelinating, or the loss of the myelin sheath insulating the nerves. When myelin degrades, conduction of signals along the nerve can be impaired or lost, and the nerve eventually withers. This leads to certain neurodegenerative disorders like multiple sclerosis and chronic inflammatory demyelinating polyneuropathy.
Alzheimer's disease
 Alzheimer's disease also known simply as Alzheimer's, is a neurodegenerative disease characterized by progressive cognitive deterioration together with declining activities of daily living and neuropsychiatric symptoms or behavioral changes. The most striking early symptom is loss of short-term memory (amnesia), which usually manifests as minor forgetfulness that becomes steadily more pronounced with illness progression, with relative preservation of older memories. As the disorder progresses, cognitive (intellectual) impairment extends to the domains of language (aphasia), skilled movements (apraxia), and recognition (agnosia), and functions such as decision-making and planning become impaired.
Alzheimer's disease also known simply as Alzheimer's, is a neurodegenerative disease characterized by progressive cognitive deterioration together with declining activities of daily living and neuropsychiatric symptoms or behavioral changes. The most striking early symptom is loss of short-term memory (amnesia), which usually manifests as minor forgetfulness that becomes steadily more pronounced with illness progression, with relative preservation of older memories. As the disorder progresses, cognitive (intellectual) impairment extends to the domains of language (aphasia), skilled movements (apraxia), and recognition (agnosia), and functions such as decision-making and planning become impaired.Charcot–Marie–Tooth disease
 It is also known as hereditary motor and sensory neuropathy (HMSN), hereditary sensorimotor neuropathy and peroneal muscular atrophy, is a heterogeneous inherited disorder of nerves (neuropathy) that is characterized by loss of muscle tissue and touch sensation, predominantly in the feet and legs but also in the hands and arms in the advanced stages of disease. Presently incurable, this disease is one of the most common inherited neurological disorders, with 37 in 100,000 affected.
It is also known as hereditary motor and sensory neuropathy (HMSN), hereditary sensorimotor neuropathy and peroneal muscular atrophy, is a heterogeneous inherited disorder of nerves (neuropathy) that is characterized by loss of muscle tissue and touch sensation, predominantly in the feet and legs but also in the hands and arms in the advanced stages of disease. Presently incurable, this disease is one of the most common inherited neurological disorders, with 37 in 100,000 affected.Neurological disorders
There Are Four Type Of Neurological Disorders
- Charcot–Marie–Tooth disease
- Alzheimer's disease
- Parkinson's disease
- Myasthenia gravis
Neurons in the brain

The number of neurons in the brain varies dramatically from species to species. One estimate puts the human brain at about 100 billion (1011) neurons and 100 trillion (1014) synapses. A lower 2012 estimate is 86 billion neurons, of which 16.3 billion are in the cerebral cortex, and 69 billion in the cerebellum. By contrast, the nematode worm Caenorhabditis elegans has just 302 neurons, making it an ideal experimental subject as scientists have been able to map all of the organism's neurons. The fruit fly Drosophila melanogaster, a common subject in biological experiments, has around 100,000 neurons and exhibits many complex behaviors. Many properties of neurons, from the type of neurotransmitters used to ion channel composition, are maintained across species, allowing scientists to study processes occurring in more complex organisms in much simpler experimental systems.
History Of Neuron
The term neuron was coined by the German anatomist Heinrich Wilhelm Waldeyer. The neuron's place as the primary functional unit of the nervous system was first recognized in the early 20th century through the work of the Spanish anatomist Santiago Ramón y Cajal. Ramón y Cajal proposed that neurons were discrete cells that communicated with each other via specialized junctions, or spaces, between cells. This became known as the neuron doctrine, one of the central tenets of modern neuroscience. To observe the structure of individual neurons, Ramón y Cajal improved a silver staining process known as Golgi's method, which had been developed by his rival, Camillo Golgi. Cajal's improvement, which involved a technique he called "double impregnation", is still in use. The silver impregnation stains are an extremely useful method for neuroanatomical investigations because, for reasons unknown, it stains a very small percentage of cells in a tissue, so one is able to see the complete micro structure of individual neurons without much overlap from other cells in the densely packed brain.
The Neuron Doctrine
The neuron doctrine is the now fundamental idea that neurons are the basic structural and functional units of the nervous system. The theory was put forward by Santiago Ramón y Cajal in the late 19th century. It held that neurons are discrete cells (not connected in a meshwork), acting as metabolically distinct units.
Later discoveries yielded a few refinements to the simplest form of the doctrine. For example, glial cells, which are not considered neurons, play an essential role in information processing. Also, electrical synapses are more common than previously thought, meaning that there are direct, cytoplasmic connections between neurons. In fact, there are examples of neurons forming even tighter coupling: the squid giant axon arises from the fusion of multiple axons.
Ramón y Cajal also postulated the Law of Dynamic Polarization, which states that a neuron receives signals at its dendrites and cell body and transmits them, as action potentials, along the axon in one direction: away from the cell body. The Law of Dynamic Polarization has important exceptions; dendrites can serve as synaptic output sites of neurons and axons can receive synaptic inputs.
All-or-none principle of Neuron
The conduction of nerve impulses is an example of an all-or-none response. In other words, if a neuron responds at all, then it must respond completely. Greater intensity of stimulation does not produce a stronger signal but can produce a higher frequency of firing. There are different types of receptor response to stimulus, slowly adapting or tonic receptors respond to steady stimulus and produce a steady rate of firing. These tonic receptors most often respond to increased intensity of stimulus by increasing their firing frequency, usually as a power function of stimulus plotted against impulses per second. This can be likened to an intrinsic property of light where to get greater intensity of a specific frequency (color) there have to be more photons, as the photons can't become "stronger" for a specific frequency.
There are a number of other receptor types that are called quickly adapting or phasic receptors, where firing decreases or stops with steady stimulus; examples include: skin when touched by an object causes the neurons to fire, but if the object maintains even pressure against the skin, the neurons stop firing. The neurons of the skin and muscles that are responsive to pressure and vibration have filtering accessory structures that aid their function.
The pacinian corpuscle is one such structure. It has concentric layers like an onion, which form around the axon terminal. When pressure is applied and the corpuscle is deformed, mechanical stimulus is transferred to the axon, which fires. If the pressure is steady, there is no more stimulus; thus, typically these neurons respond with a transient depolarization during the initial deformation and again when the pressure is removed, which causes the corpuscle to change shape again. Other types of adaptation are important in extending the function of a number of other neurons.
Neural coding
 Neural coding is concerned with how sensory and other information is represented in the brain by neurons. The main goal of studying neural coding is to characterize the relationship between the stimulus and the individual or ensemble neuronal responses, and the relationships amongst the electrical activities of the neurons within the ensemble. It is thought that neurons can encode both digital and analog information.
Neural coding is concerned with how sensory and other information is represented in the brain by neurons. The main goal of studying neural coding is to characterize the relationship between the stimulus and the individual or ensemble neuronal responses, and the relationships amongst the electrical activities of the neurons within the ensemble. It is thought that neurons can encode both digital and analog information.Mechanisms for propagating action potentials Of Neuron
In 1937, John Zachary Young suggested that the squid giant axon could be used to study neuronal electrical properties. Being larger than but similar in nature to human neurons, squid cells were easier to study. By inserting electrodes into the giant squid axons, accurate measurements were made of the membrane potential.
The cell membrane of the axon and soma contain voltage-gated ion channels that allow the neuron to generate and propagate an electrical signal (an action potential). These signals are generated and propagated by charge-carrying ions including sodium (Na+), potassium (K+), chloride (Cl-), and calcium (Ca2+).
There are several stimuli that can activate a neuron leading to electrical activity, including pressure, stretch, chemical transmitters, and changes of the electric potential across the cell membrane. Stimuli cause specific ion-channels within the cell membrane to open, leading to a flow of ions through the cell membrane, changing the membrane potential.
Thin neurons and axons require less metabolic expense to produce and carry action potentials, but thicker axons convey impulses more rapidly. To minimize metabolic expense while maintaining rapid conduction, many neurons have insulating sheaths of myelin around their axons. The sheaths are formed by glial cells: oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system. The sheath enables action potentials to travel faster than in unmyelinated axons of the same diameter, whilst using less energy. The myelin sheath in peripheral nerves normally runs along the axon in sections about 1 mm long, punctuated by unsheathed nodes of Ranvier, which contain a high density of voltage-gated ion channels. Multiple sclerosis is a neurological disorder that results from demyelination of axons in the central nervous system.
Some neurons do not generate action potentials, but instead generate a graded electrical signal, which in turn causes graded neurotransmitter release. Such nonspiking neurons tend to be sensory neurons or interneurons, because they cannot carry signals long distances.
Connectivity Of Neuron
 Neurons communicate with one another via synapses, where the axon terminal or en passant boutons (terminals located along the length of the axon) of one cell impinges upon another neuron's dendrite, soma or, less commonly, axon. Neurons such as Purkinje cells in the cerebellum can have over 1000 dendritic branches, making connections with tens of thousands of other cells; other neurons, such as the magnocellular neurons of the supraoptic nucleus, have only one or two dendrites, each of which receives thousands of synapses. Synapses can be excitatory or inhibitory and either increase or decrease activity in the target neuron. Some neurons also communicate via electrical synapses, which are direct, electrically conductive junctions between cells.
Neurons communicate with one another via synapses, where the axon terminal or en passant boutons (terminals located along the length of the axon) of one cell impinges upon another neuron's dendrite, soma or, less commonly, axon. Neurons such as Purkinje cells in the cerebellum can have over 1000 dendritic branches, making connections with tens of thousands of other cells; other neurons, such as the magnocellular neurons of the supraoptic nucleus, have only one or two dendrites, each of which receives thousands of synapses. Synapses can be excitatory or inhibitory and either increase or decrease activity in the target neuron. Some neurons also communicate via electrical synapses, which are direct, electrically conductive junctions between cells.
In a chemical synapse, the process of synaptic transmission is as follows: when an action potential reaches the axon terminal, it opens voltage-gated calcium channels, allowing calcium ions to enter the terminal. Calcium causes synaptic vesicles filled with neurotransmitter molecules to fuse with the membrane, releasing their contents into the synaptic cleft. The neurotransmitters diffuse across the synaptic cleft and activate receptors on the postsynaptic neuron.
The human brain has a huge number of synapses. Each of the 1011 (one hundred billion) neurons has on average 7,000 synaptic connections to other neurons. It has been estimated that the brain of a three-year-old child has about 1015 synapses (1 quadrillion). This number declines with age, stabilizing by adulthood. Estimates vary for an adult, ranging from 1014 to 5 x 1014 synapses (100 to 500 trillion).
Structural classification Of Neurons
Structural classification Of Neurons
Different kinds of neurons:
1 Unipolar neuron
2 Bipolar neuron
4 Pseudounipolar neuron
Most neurons can be anatomically characterized as:
Unipolar or pseudounipolar: dendrite and axon emerging from same process.
Bipolar: axon and single dendrite on opposite ends of the soma.
Multipolar: more than two dendrites:
Golgi I: neurons with long-projecting axonal processes; examples are pyramidal cells, Purkinje cells, and anterior horn cells.
Golgi II: neurons whose axonal process projects locally; the best example is the granule cell.
Other
Furthermore, some unique neuronal types can be identified according to their location in the nervous system and distinct shape. Some examples are:
Basket cells, interneurons that form a dense plexus of terminals around the soma of target cells, found in the cortex and cerebellum.
Betz cells, large motor neurons.
Medium spiny neurons, most neurons in the corpus striatum.
Purkinje cells, huge neurons in the cerebellum, a type of Golgi I multipolar neuron.
Pyramidal cells, neurons with triangular soma, a type of Golgi I.
Renshaw cells, neurons with both ends linked to alpha motor neurons.
Granule cells, a type of Golgi II neuron.
Anterior horn cells, motoneurons located in the spinal cord.
Spindle cells, interneurons that connect widely separated areas of the brain
Serotonergic neurons
Serotonin,(5-Hydroxytryptamine, 5-HT), can act as excitatory or inhibitory. Of the four 5-HT receptor classes, 3 are GPCR and 1 is ligand gated cation channel. Serotonin is synthesized from tryptophan by tryptophan hydroxylase, and then further by aromatic acid decarboxylase. A lack of 5-HT at postsynaptic neurons has been linked to depression. Drugs that block the presynaptic serotonin transporter are used for treatment, such as Prozac and Zoloft.
Dopaminergic neurons
.
Glutamatergic neurons
Glutamate is one of two primary excitatory amino acids, the other being Aspartate. Glutamate receptors are one of four categories, three of which are ligand-gated ion channels and one of which is a G-protein coupled receptor (often referred to as GPCR).
- AMPA and Kainate receptors both function as cation channels permeable to Na+ cation channels mediating fast excitatory synaptic transmission
- NMDA receptors are another cation channel that is more permeable to Ca2+. The function of NMDA receptors is dependant on Glycine receptor binding as a co-agonist within the channel pore. NMDA receptors do not function without both ligands present.
- Metabotropic receptors, GPCRs modulate synaptic transmission and postsynaptic excitability.
GABAergic neurons
gamma aminobutyric acid. GABA is one of two neuroinhibitors in the CNS, the other being Glycine. GABA has a homologous function to ACh, gating anion channels that allow Cl- ions to enter the post synaptic neuron. Cl- causes hyperpolarization within the neuron, decreasing the probability of an action potential firing as the voltage becomes more negative (recall that for an action potential to fire, a positive voltage threshold must be reached).
Cholinergic neurons
cetylcholine. Acetylcholine is released from presynaptic neurons into the synaptic cleft. It acts as a ligand for both ligand-gated ion channels and metabotropic (GPCRs) muscarinic receptors. Nicotinic receptors, are pentameric ligand-gated ion channels composed of alpha and beta subunits that bind nicotine. Ligand binding opens the channel causing influx of Na+ depolarization and increases the probability of presynaptic neurotransmitter release.
Classification by neurotransmitter production
There Are Five Classification By Neurotransmitter Production
Discharge patterns Neuron
Neurons can be classified according to their electrophysiological characteristics: Tonic or regular spiking. Some neurons are typically constantly (or tonically) active. Example: interneurons in neurostriatum. Phasic or bursting. Neurons that fire in bursts are called phasic. Fast spiking. Some neurons are notable for their high firing rates, for example some types of cortical inhibitory interneurons, cells in globus pallidus, retinal ganglion cells.
Action On Other Neurons
A neuron affects other neurons by releasing a neurotransmitter that binds to chemical receptors. The effect upon the postsynaptic neuron is determined not by the presynaptic neuron or by the neurotransmitter, but by the type of receptor that is activated. A neurotransmitter can be thought of as a key, and a receptor as a lock: the same type of key can here be used to open many different types of locks. Receptors can be classified broadly as excitatory (causing an increase in firing rate), inhibitory (causing a decrease in firing rate), or modulatory (causing long-lasting effects not directly related to firing rate).
The two most common neurotransmitters in the brain, glutamate and GABA, have actions that are largely consistent. Glutamate acts on several different types of receptors, and have effects that are excitatory at ionotropic receptors and a modulatory effect at metabotropic receptors. Similarly GABA acts on several different types of receptors, but all of them have effects (in adult animals, at least) that are inhibitory. Because of this consistency, it is common for neuroscientists to simplify the terminology by referring to cells that release glutamate as "excitatory neurons," and cells that release GABA as "inhibitory neurons." Since over 90% of the neurons in the brain release either glutamate or GABA, these labels encompass the great majority of neurons. There are also other types of neurons that have consistent effects on their targets, for example "excitatory" motor neurons in the spinal cord that release acetylcholine, and "inhibitory" spinal neurons that release glycine.
The distinction between excitatory and inhibitory neurotransmitters is not absolute, however. Rather, it depends on the class of chemical receptors present on the postsynaptic neuron. In principle, a single neuron, releasing a single neurotransmitter, can have excitatory effects on some targets, inhibitory effects on others, and modulatory effects on others still. For example, photoreceptor cells in the retina constantly release the neurotransmitter glutamate in the absence of light. So-called OFF bipolar cells are, like most neurons, excited by the released glutamate. However, neighboring target neurons called ON bipolar cells are instead inhibited by glutamate, because they lack the typical ionotropic glutamate receptors and instead express a class of inhibitory metabotropic glutamate receptors. When light is present, the photoreceptors cease releasing glutamate, which relieves the ON bipolar cells from inhibition, activating them; this simultaneously removes the excitation from the OFF bipolar cells, silencing them.
It is possible to identify the type of inhibitory effect a presynaptic neuron will have on a postsynaptic neuron, based on the proteins the presynaptic neuron expresses. Parvalbumin-expressing neurons typically dampen the output signal of the postsynaptic neuron in the visual cortex, whereas somatostatin-expressing neurons typically block dendritic inputs to the postsynaptic neuron.
Direction Neurons
Afferent neurons convey information from tissues and organs into the central nervous system and are sometimes also called sensory neurons.
Efferent neurons transmit signals from the central nervous system to the effector cells and are sometimes called motor neurons.
Interneurons connect neurons within specific regions of the central nervous system.
Afferent and efferent also refer generally to neurons that, respectively, bring information to or send information from the brain region.
Functional classification Neurons
Functional classification Neurons
- Direction neurons
- Action on other neurons
- Discharge patterns neurons
Structural classification Of Neurons
Different kinds of neurons:
1 Unipolar neuron
2 Bipolar neuron
4 Pseudounipolar neuron
Most neurons can be anatomically characterized as:
Unipolar or pseudounipolar: dendrite and axon emerging from same process.
Bipolar: axon and single dendrite on opposite ends of the soma.
Multipolar: more than two dendrites:
Golgi I: neurons with long-projecting axonal processes; examples are pyramidal cells, Purkinje cells, and anterior horn cells.
Golgi II: neurons whose axonal process projects locally; the best example is the granule cell.
Other
Furthermore, some unique neuronal types can be identified according to their location in the nervous system and distinct shape. Some examples are:
Basket cells, interneurons that form a dense plexus of terminals around the soma of target cells, found in the cortex and cerebellum.
Betz cells, large motor neurons.
Medium spiny neurons, most neurons in the corpus striatum.
Purkinje cells, huge neurons in the cerebellum, a type of Golgi I multipolar neuron.
Pyramidal cells, neurons with triangular soma, a type of Golgi I.
Renshaw cells, neurons with both ends linked to alpha motor neurons.
Granule cells, a type of Golgi II neuron.
Anterior horn cells, motoneurons located in the spinal cord.
Spindle cells, interneurons that connect widely separated areas of the brain
Classes Of Neurons
Neurons exist in a number of different shapes and sizes and can be classified by their morphology and function. The anatomist Camillo Golgi grouped neurons into two types; type I with long axons used to move signals over long distances and type II with short axons, which can often be confused with dendrites. Type I cells can be further divided by where the cell body or soma is located. The basic morphology of type I neurons, represented by spinal motor neurons, consists of a cell body called the soma and a long thin axon covered by the myelin sheath. Around the cell body is a branching dendritic tree that receives signals from other neurons. The end of the axon has branching terminals (axon terminal) that release neurotransmitters into a gap called the synaptic cleft between the terminals and the dendrites of the next neuron.
Histology and internal structure Of Neurons
Nerve cell bodies stained with basophilic dyes show numerous microscopic clumps of Nissl substance (named after German psychiatrist and neuropathologist Franz Nissl, 1860–1919), which consists of rough endoplasmic reticulum and associated ribosomal RNA. The prominence of the Nissl substance can be explained by the fact that nerve cells are metabolically very active, and hence are involved in large amounts of protein synthesis.
The cell body of a neuron is supported by a complex meshwork of structural proteins called neurofilaments, which are assembled into larger neurofibrils. Some neurons also contain pigment granules, such as neuromelanin (a brownish-black pigment, byproduct of synthesis of catecholamines) and lipofuscin (yellowish-brown pigment that accumulates with age).
There are different internal structural characteristics between axons and dendrites. Typical axons almost never contain ribosomes, except some in the initial segment. Dendrites contain granular endoplasmic reticulum or ribosomes, with diminishing amounts with distance from the cell body.
Anatomy and Histology Of Neurons
Neurons are highly specialized for the processing and transmission of cellular signals. Given the diversity of functions performed by neurons in different parts of the nervous system, there is, as expected, a wide variety in the shape, size, and electrochemical properties of neurons. For instance, the soma of a neuron can vary from 4 to 100 micrometers in diameter.
 The soma is the central part of the neuron. It contains the nucleus of the cell, and therefore is where most protein synthesis occurs. The nucleus ranges from 3 to 18 micrometers in diameter.
The soma is the central part of the neuron. It contains the nucleus of the cell, and therefore is where most protein synthesis occurs. The nucleus ranges from 3 to 18 micrometers in diameter.
The dendrites of a neuron are cellular extensions with many branches, and metaphorically this overall shape and structure is referred to as a dendritic tree. This is where the majority of input to the neuron occurs via the dendritic spine.
The axon is a finer, cable-like projection that can extend tens, hundreds, or even tens of thousands of times the diameter of the soma in length. The axon carries nerve signals away from the soma (and also carries some types of information back to it). Many neurons have only one axon, but this axon may—and usually will—undergo extensive branching, enabling communication with many target cells. The part of the axon where it emerges from the soma is called the axon hillock. Besides being an anatomical structure, the axon hillock is also the part of the neuron that has the greatest density of voltage-dependent sodium channels. This makes it the most easily excited part of the neuron and the spike initiation zone for the axon: in electrophysiological terms it has the most negative action potential threshold. While the axon and axon hillock are generally involved in information outflow, this region can also receive input from other neurons.
The axon terminal contains synapses, specialized structures where neurotransmitter chemicals are released to communicate with target neurons.
Although the canonical view of the neuron attributes dedicated functions to its various anatomical components, dendrites and axons often act in ways contrary to their so-called main function.
Axons and dendrites in the central nervous system are typically only about one micrometer thick, while some in the peripheral nervous system are much thicker. The soma is usually about 10–25 micrometers in diameter and often is not much larger than the cell nucleus it contains. The longest axon of a human motoneuron can be over a meter long, reaching from the base of the spine to the toes. Sensory neurons have axons that run from the toes to the dorsal columns, over 1.5 meters in adults. Giraffes have single axons several meters in length running along the entire length of their necks. Much of what is known about axonal function comes from studying the squid giant axon, an ideal experimental preparation because of its relatively immense size (0.5–1 millimeters thick, several centimeters long).
Fully differentiated neurons are permanently postmitotic; however, recent research shows that additional neurons throughout the brain can originate from neural stem cells found throughout the brain but in particularly high concentrations in the subventricular zone and subgranular zone through the process of neurogenesis.
Neuron
A neuron (/ˈnjʊərɒn/ nyewr-on or /ˈnʊərɒn/ newr-on; also known as a neuron or nerve cell) is an electrically excitable cell that processes and transmits information through electrical and chemical signals. A chemical signal occurs via a synapse, a specialized connection with other cells. Neurons connect to each other to form neural networks. Neurons are the core components of the nervous system, which includes the brain, spinal cord, and peripheral ganglia. A number of specialized types of neurons exist: sensory neurons respond to touch, sound, light and numerous other stimuli affecting cells of the sensory organs that then send signals to the spinal cord and brain. Motor neurons receive signals from the brain and spinal cord, cause muscle contractions, and affect glands. Interneurons connect neurons to other neurons within the same region of the brain or spinal cord.
A typical neuron possesses a cell body (often called the soma), dendrites, and an axon. Dendrites are thin structures that arise from the cell body, often extending for hundreds of micrometres and branching multiple times, giving rise to a complex "dendritic tree". An axon is a special cellular extension that arises from the cell body at a site called the axon hillock and travels for a distance, as far as 1 meter in humans or even more in other species. The cell body of a neuron frequently gives rise to multiple dendrites, but never to more than one axon, although the axon may branch hundreds of times before it terminates. At the majority of synapses, signals are sent from the axon of one neuron to a dendrite of another. There are, however, many exceptions to these rules: neurons that lack dendrites, neurons that have no axon, synapses that connect an axon to another axon or a dendrite to another dendrite, etc.
All neurons are electrically excitable, maintaining voltage gradients across their membranes by means of metabolically driven ion pumps, which combine with ion channels embedded in the membrane to generate intracellular-versus-extracellular concentration differences of ions such as sodium, potassium, chloride, and calcium. Changes in the cross-membrane voltage can alter the function of voltage-dependent ion channels. If the voltage changes by a large enough amount, an all-or-none electrochemical pulse called an action potential is generated, which travels rapidly along the cell's axon, and activates synaptic connections with other cells when it arrives.
Neurons do not undergo cell division. In most cases, neurons are generated by special types of stem cells. Astrocytes, a type of glial cell, have also been observed to turn into neurons by virtue of the stem cell characteristic pluripotency. In humans, neurogenesis largely ceases during adulthood—but in two brain areas, the hippocampus and olfactory bulb, there is strong evidence for generation of substantial numbers of new neurons.
Definition Of Neuron
 A neuron is a specialized type of cell found in the bodies of most animals (all members of the group Eumetazoa). Only sponges and a few other simpler animals have no neurons. The features that define a neuron are electrical excitability and the presence of synapses, which are complex membrane junctions that transmit signals to other cells. The body's neurons, plus the glial cells that give them structural and metabolic support, together constitute the nervous system. In vertebrates, the majority of neurons belong to the central nervous system, but some reside in peripheral ganglia, and many sensory neurons are situated in sensory organs such as the retina and cochlea.
A neuron is a specialized type of cell found in the bodies of most animals (all members of the group Eumetazoa). Only sponges and a few other simpler animals have no neurons. The features that define a neuron are electrical excitability and the presence of synapses, which are complex membrane junctions that transmit signals to other cells. The body's neurons, plus the glial cells that give them structural and metabolic support, together constitute the nervous system. In vertebrates, the majority of neurons belong to the central nervous system, but some reside in peripheral ganglia, and many sensory neurons are situated in sensory organs such as the retina and cochlea.
Although neurons are very diverse and there are exceptions to nearly every rule, it is convenient to begin with a schematic description of the structure and function of a "typical" neuron. A typical neuron is divided into three parts: the soma or cell body, dendrites, and axon. The soma is usually compact; the axon and dendrites are filaments that extrude from it. Dendrites typically branch profusely, getting thinner with each branching, and extending their farthest branches a few hundred micrometres from the soma. The axon leaves the soma at a swelling called the axon hillock, and can extend for great distances, giving rise to hundreds of branches. Unlike dendrites, an axon usually maintains the same diameter as it extends. The soma may give rise to numerous dendrites, but never to more than one axon. Synaptic signals from other neurons are received by the soma and dendrites; signals to other neurons are transmitted by the axon. A typical synapse, then, is a contact between the axon of one neuron and a dendrite or soma of another. Synaptic signals may be excitatory or inhibitory. If the net excitation received by a neuron over a short period of time is large enough, the neuron generates a brief pulse called an action potential, which originates at the soma and propagates rapidly along the axon, activating synapses onto other neurons as it goes.
Many neurons fit the foregoing schema in every respect, but there are also exceptions to most parts of it. There are no neurons that lack a soma, but there are neurons that lack dendrites, and others that lack an axon. Furthermore, in addition to the typical axodendritic and axosomatic synapses, there are axoaxonic (axon-to-axon) and dendrodendritic (dendrite-to-dendrite) synapses.
The key to neural function is the synaptic signaling process, which is partly electrical and partly chemical. The electrical aspect depends on properties of the neuron's membrane. Like all animal cells, every neuron is surrounded by a plasma membrane, a bilayer of lipid molecules with many types of protein structures embedded in it. A lipid bilayer is a powerful electrical insulator, but in neurons, many of the protein structures embedded in the membrane are electrically active. These include ion channels that permit electrically charged ions to flow across the membrane, and ion pumps that actively transport ions from one side of the membrane to the other. Most ion channels are permeable only to specific types of ions. Some ion channels are voltage gated, meaning that they can be switched between open and closed states by altering the voltage difference across the membrane. Others are chemically gated, meaning that they can be switched between open and closed states by interactions with chemicals that diffuse through the extracellular fluid. The interactions between ion channels and ion pumps produce a voltage difference across the membrane, typically a bit less than 1/10 of a volt at baseline. This voltage has two functions: first, it provides a power source for an assortment of voltage-dependent protein machinery that is embedded in the membrane; second, it provides a basis for electrical signal transmission between different parts of the membrane.
Neurons communicate by chemical and electrical synapses in a process known as synaptic transmission. The fundamental process that triggers synaptic transmission is the action potential, a propagating electrical signal that is generated by exploiting the electrically excitable membrane of the neuron. This is also known as a wave of depolarization
Structure Of Neuron
 A neuron is a specialized type of cell found in the bodies of most animals (all members of the group Eumetazoa). Only sponges and a few other simpler animals have no neurons. The features that define a neuron are electrical excitability and the presence of synapses, which are complex membrane junctions that transmit signals to other cells. The body's neurons, plus the glial cells that give them structural and metabolic support, together constitute the nervous system. In vertebrates, the majority of neurons belong to the central nervous system, but some reside in peripheral ganglia, and many sensory neurons are situated in sensory organs such as the retina and cochlea.
A neuron is a specialized type of cell found in the bodies of most animals (all members of the group Eumetazoa). Only sponges and a few other simpler animals have no neurons. The features that define a neuron are electrical excitability and the presence of synapses, which are complex membrane junctions that transmit signals to other cells. The body's neurons, plus the glial cells that give them structural and metabolic support, together constitute the nervous system. In vertebrates, the majority of neurons belong to the central nervous system, but some reside in peripheral ganglia, and many sensory neurons are situated in sensory organs such as the retina and cochlea.
Although neurons are very diverse and there are exceptions to nearly every rule, it is convenient to begin with a schematic description of the structure and function of a "typical" neuron. A typical neuron is divided into three parts: the soma or cell body, dendrites, and axon. The soma is usually compact; the axon and dendrites are filaments that extrude from it. Dendrites typically branch profusely, getting thinner with each branching, and extending their farthest branches a few hundred micrometres from the soma. The axon leaves the soma at a swelling called the axon hillock, and can extend for great distances, giving rise to hundreds of branches. Unlike dendrites, an axon usually maintains the same diameter as it extends. The soma may give rise to numerous dendrites, but never to more than one axon. Synaptic signals from other neurons are received by the soma and dendrites; signals to other neurons are transmitted by the axon. A typical synapse, then, is a contact between the axon of one neuron and a dendrite or soma of another. Synaptic signals may be excitatory or inhibitory. If the net excitation received by a neuron over a short period of time is large enough, the neuron generates a brief pulse called an action potential, which originates at the soma and propagates rapidly along the axon, activating synapses onto other neurons as it goes.
Many neurons fit the foregoing schema in every respect, but there are also exceptions to most parts of it. There are no neurons that lack a soma, but there are neurons that lack dendrites, and others that lack an axon. Furthermore, in addition to the typical axodendritic and axosomatic synapses, there are axoaxonic (axon-to-axon) and dendrodendritic (dendrite-to-dendrite) synapses.
The key to neural function is the synaptic signaling process, which is partly electrical and partly chemical. The electrical aspect depends on properties of the neuron's membrane. Like all animal cells, every neuron is surrounded by a plasma membrane, a bilayer of lipid molecules with many types of protein structures embedded in it. A lipid bilayer is a powerful electrical insulator, but in neurons, many of the protein structures embedded in the membrane are electrically active. These include ion channels that permit electrically charged ions to flow across the membrane, and ion pumps that actively transport ions from one side of the membrane to the other. Most ion channels are permeable only to specific types of ions. Some ion channels are voltage gated, meaning that they can be switched between open and closed states by altering the voltage difference across the membrane. Others are chemically gated, meaning that they can be switched between open and closed states by interactions with chemicals that diffuse through the extracellular fluid. The interactions between ion channels and ion pumps produce a voltage difference across the membrane, typically a bit less than 1/10 of a volt at baseline. This voltage has two functions: first, it provides a power source for an assortment of voltage-dependent protein machinery that is embedded in the membrane; second, it provides a basis for electrical signal transmission between different parts of the membrane.
Neurons communicate by chemical and electrical synapses in a process known as synaptic transmission. The fundamental process that triggers synaptic transmission is the action potential, a propagating electrical signal that is generated by exploiting the electrically excitable membrane of the neuron. This is also known as a wave of depolarization


























